AIM 1
User-Centered Design Approach
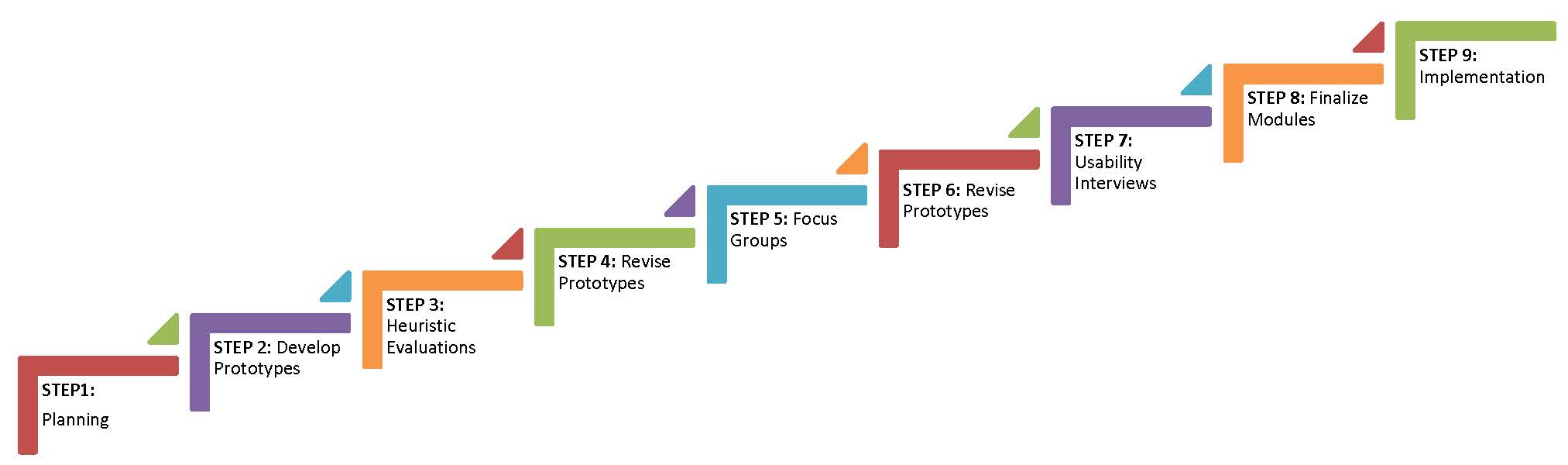
For designing healthcare systems, such as electronic medical record systems, Kushniruk & Patel (2004) suggest using several stages with iterative cycles that return to prior stages for further design, development and evaluation. The planning stages of this approach include understanding the users, the environment, the tasks, functions of the interface, and optimal display of information (Johnson et al. 2005; Stone et al. 2005). Prototype evaluation can include heuristic testing, individual user testing, pluralistic or cognitive walkthroughs, usability inspections, and others, with data collection methods including observation, interviews, think-aloud protocols, surveys, and video analysis (Lee 1999; Taylor et al. 2011). Thus, our project employs a User-Centered Design approach with the following steps:

STEP 1: Planning. Collect information about users, site needs, consent requirements, and support needs of the target population.
STEP 2: Develop Prototype. Apply principles of cognitive load to "chunk" the consent documents into one to two concepts per slide. Chunking text helps reduce the amount of information presented at once and thus facilitates learning (Clark & Mayer 2008; Mayer 2009). Add graphics to slides where a concept can be depicted visually. Include interactive questions with salient feedback to reinforce or clarify concepts.
STEP 3. Heuristic Evaluation. Biobank staff (e.g. site investigators, biobank directors, research coordinators) and external project consultants evaluate the prototypes developed in Step 2 by completing an e-survey based on formative evaluation recommendations for multimedia (e.g., Alessi & Trollip 2001).
STEP 4: Revise Prototype. Revise prototype based on feedback from heuristic evaluation. Revisions to the prototype will be made for feedback categorized as critical and, if possible, important. Revisions based on desirable status wil be made if resources are available and the modification fits the initial design analysis.
STEP 5: Focus Group Evaluation. Conduct focus groups at each site (Emory, Montefiore, and Northwestern) to learn from prospective users what they like and dislike about the prototype, and what they suggest as possible improvements. The focus groups are aimed at understanding how the prototype compares to the paper document for effectiveness at promoting understanding, the appropriateness and utility of the graphics, and the clarity and effectiveness of the interactive questions.
STEP 6: Revise Prototype. Revise prototype based on feedback from the focus groups. Revisions to the prototype will be made for feedback categorized as critical and, if possible, important. Revisions based on desirable status wil be made if resources are available and the modification fits the initial design analysis.
STEP 7: Usability Interviews. Conducted with 3-5 individuals from the target user population at each site (Emory, Montefiore, and Northwestern). Individuals are asked to go through the prototype for their site using a think-aloud protocol, while being video-recorded and observed by a project research assistant who wil be trained as a usability tester. The observer looks for potential miscommunications; errors using the interface; strengths and weaknesses in the graphics, interactive questions, and the interface controls; and indications of satisfaction or dissatisfaction in the user. Once the user has completed the prototype, the observer conducts a brief interview with the user to explore issues and answer any questions.
STEP 8: Revise Final Prototype. Revise prototype based on feedback from the usability interviews to create the final version for each site. Revisions to the prototype will be made for feedback categorized as critical and, if possible, important. Revisions based on desirable status wil be made if resources are available and the modification fits the initial design analysis.
STEP 9: Implementation. Implementation of the final prototype in a randomized controlled trial (Aim2).
AIM 2
Randomized Controlled Trial
The objective of Aim 2 is to establish the effectiveness (capacity to promote understanding) and efficiency (staff time spent in the consent process) of interactive multimedia (IM) compared to standard face-to-face (F2F) consenting methods. Participant confidence in their understanding, satisfaction and trust with respect to the consent process and biobank-supported research will also be compared. If IM consent is demonstrably more effective in promoting participants’ understanding, confidence, satisfaction and trust compared to standard F2F consent processes, the project will have demonstrated the utility of interactive multimedia as a means of improving participants’ understanding and their perception of biobanks and genetic research.
The IM consent modules developed in Aim 1 will be used in the study’s three partner biobanks (at Emory, Montefiore, and Northwestern).
Each site will enroll and collect complete data from 200 English-speaking participants. Montefiore will enroll an additional 100 Spanish-speaking participants.
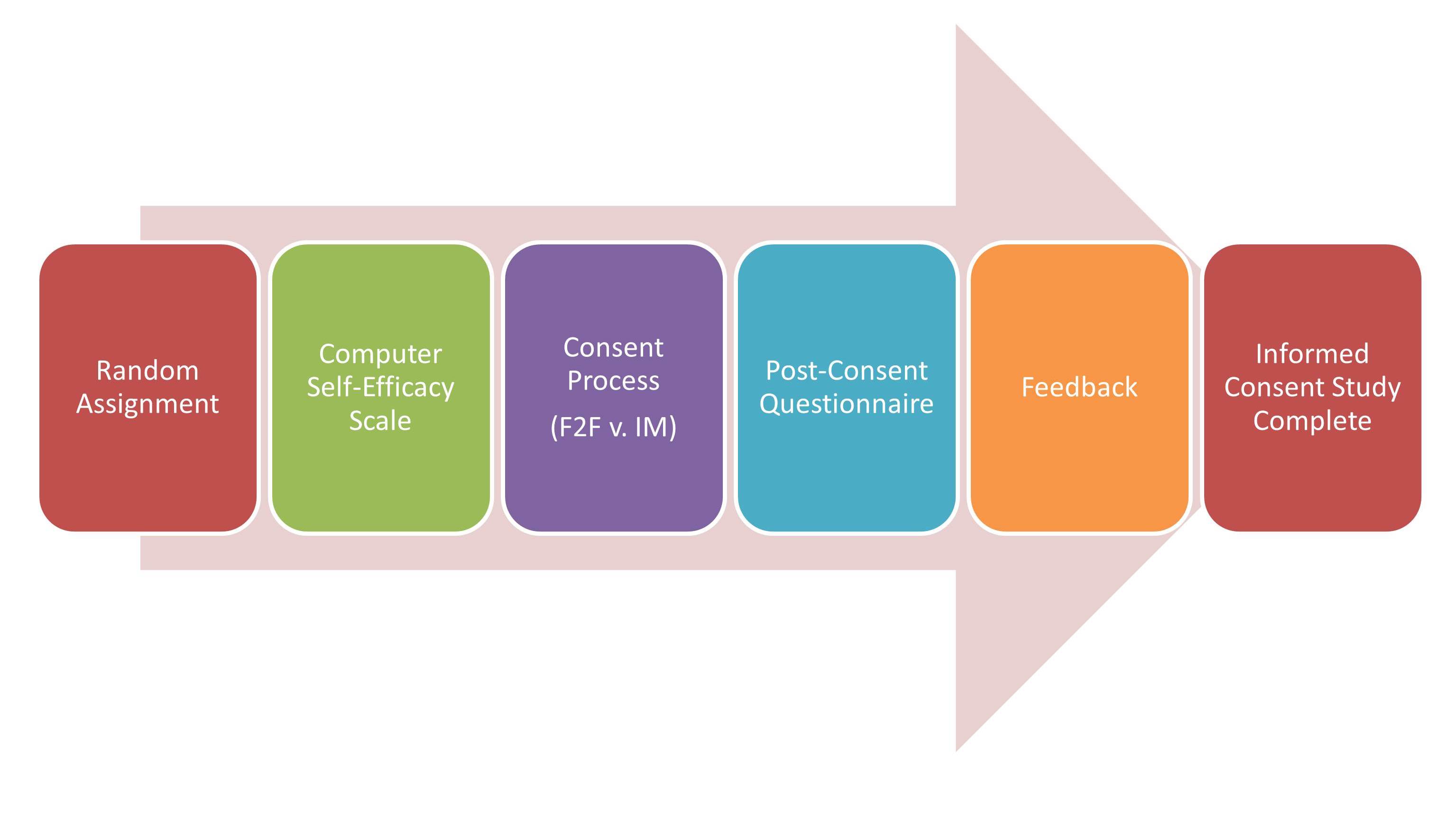
STEP 1. Random Assignment. Participants are randomly assigned to either the IM or standard F2F conditions.
STEP 2. Computer Self-Efficacy Scale. Prior to the IM and F2F informed consent processes, participants are asked to complete this brief, 10 question assessment about how confident they feel using computers to perform certain tasks.
STEP 3. Consent Process. Patients randomly assigned to receive the standard F2F condition are led through that condition by the RA/research coordinator who normally does the biobank’s F2F consent. The study did not request changes in the biobank’s current F2F processes, other than the completion of the computer self-efficacy scale and the post-consent questionnaire. For patients randomized to the IM condition, an RA hired specifically for this project provides the IM consent module. In the IM consent module, text is displayed on a computer screen, both as written text and audio narration of the text, supported with graphics. Participants are asked and must respond to scripted questions at the end of each section and receive scripted feedback based on their responses in order to correct misconceptions. Participants control the speed of the display and the volume. The RA is available to answer questions at any time.
STEP 4. Post-Consent Questionnaire. After completing either the IM or F2F consent process, participants complete the Post-Consent Questionnaire to assess their understanding, confidence in their understanding, satisfaction with the consent process, their trust in biobanks, and to collect demographic information.
STEP 5. Feedback. The post-consent assessment instrument identifies questions answered incorrectly and presents a page with feedback that provides the correct answers and corrective feedback for possible misconceptions.